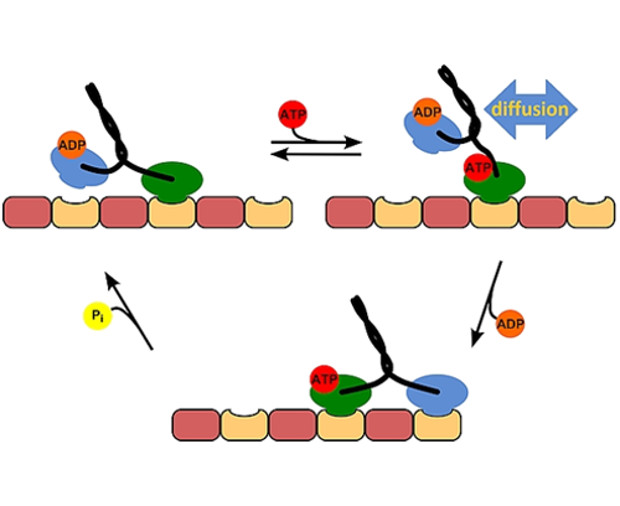
Kinesin is a motor protein, responsible for transporting cargo across cells in our bodies. It can pull a load
with a force of several piconewtons. However, according to our recent results, developed in
collaboration with the prof. Diez group from B-CUBE Dresden, kinesin can be stopped by a moderate
increase the viscosity of its environment. The drag force when kinesin stalls is less than 0.001
piconewton – thousands of times less than expected!
The kinesin engine comprises two “heads” connected by a short, flexible linker. It moves along
microtubules – the cellular scaffolding – in a step-by-step manner. The key issue here is synchronization
between the two heads, so that kinesin can make steps without falling off its path.
By increasing viscosity, we slowed down diffusion of the heads. Such slight shift of equilibrium in one of
the reactions in the stepping cycle was enough to sabotage the whole process. No previous study noted
this, since they all used long polymers to adjust viscosity, so the small kinesin head could still easily move
between them. This underlines the biophysical importance of the length-scale-dependent viscosity
model developed in our group. Our current findings support the theory that kinesin is actually powered
by Brownian motion, while ATP – the cellular fuel – is rather used to keep the direction and
synchronization of steps. Since the stall viscosity is only slightly higher than what is usually observed in
mammalian cells, we may even speculate about the regulatory role of this simple parameter in the
intercellular transport system.